In the realm of physics, the study of mixtures presents profound implications for understanding complex systems, including biological cells. Researchers from São Paulo State University (UNESP) have leveraged principles derived from condensed matter physics to explore an innovative model of protein compartmentalization within cells. This examination parallels the dynamics observed in classical Griffiths phases, which manifest in magnetic systems characterized by spatial randomness and emergent behavior in material substances. This groundbreaking research opens new avenues for comprehending cellular behavior and its implications for biology and medicine.
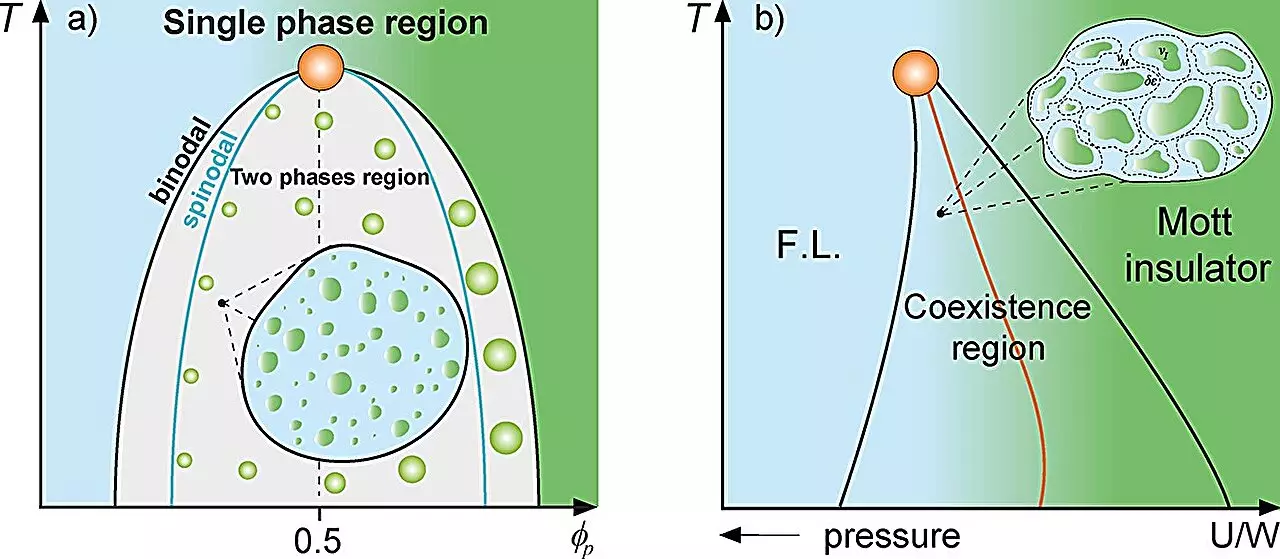
The Griffiths phase is a specific state found in disordered magnetic systems where unique regions (or “rare regions”) exhibit different magnetic properties than their surrounding material. By drawing parallels with this phenomenon, the UNESP team, led by Professor Mariano de Souza and Ph.D. candidate Lucas Squillante, identified comparable dynamics in living cells, particularly in the formation of protein droplets. These droplets arise from liquid-liquid phase separation, an essential cellular process that impacts numerous cellular functions.
In their publication, the researchers noted that the dynamics of proteins are significantly tempered in the vicinity of the critical phase separation threshold, analogous to how magnetic systems behave at the Griffiths phase. The identification of these ‘rare regions’ within cells signifies that, like their physical counterparts, certain cellular behaviors emerge unpredictably due to underlying physical principles. This representation illustrates that cells not only house structures for biochemical reactions but also exhibit complex behaviors governed by physical laws.
One of the more intriguing hypotheses proposed by Souza and his colleagues is the potential connection between their findings and the origins of life itself. The research alludes to classical theories posited by Aleksandr Oparin regarding the emergence of life through coacervates—aggregates of organic molecules that facilitated early biological processes. The Griffiths-like cellular phase described in their work may shed light on how early life forms could have developed slower dynamics, which would be essential for stability and, consequently, evolution.
This relationship also touches on the significant aspect of homochirality—the prevalence of one chiral form of molecules over others in biological systems. The emergence of homochirality is essential for the uniformity of biological processes, and understanding how the proposed Griffiths-like phase influences protein dynamics could refine our grasp of these foundational phenomena.
The study highlights the relevance of phase separation in various pathological states, particularly emphasizing its implications for tumorigenesis and neurodegenerative diseases. Understanding how proteins compartmentalize and their resulting dynamics may unlock novel therapeutic strategies. For example, the interplay between liquid-liquid phase separation and diseases such as cataracts, where visual impairment stems from phase separation in the retina, signifies the necessity of interdisciplinary research bridging physics, biology, and medicine.
Furthermore, the collaboration with clinical researchers provides a multifaceted view of disease mechanisms. Associate Professor Marcos Minicucci notes that the compartmentalization of proteins involved in diseases could rationalize mutation processes and help inform treatments. Such interdisciplinary efforts underscore the potential for novel insights into disease management through the lens of physical phenomena governing biological systems.
The findings by Souza’s team pave the way for further research into the biological ramifications of phase separation. The described Griffiths-like cellular phase offers a framework for exploring not only basic cellular functions but the complex interrelations between protein dynamics and pathophysiological conditions. Future studies may clarify how manipulating phase behavior could inform interventions and treatments in clinical settings, particularly as evidenced by the intriguing relationships observed in cancer therapy.
As our understanding deepens, the cross-pollination of fields within science will likely yield richer insights into cell dynamics, disease process elucidation, and potentially revolutionary therapeutic applications. The collaboration among professionals across a range of disciplines—from biophysics to clinical medicine—serves as a testament to the importance of integrative approaches in addressing contemporary scientific challenges.
The research conducted by the UNESP team not only broadens our understanding of protein behavior within cells but also invites a reinterpretation of the dynamics underpinning life itself, opening unexplored avenues for future inquiry and application in biology and medicine.