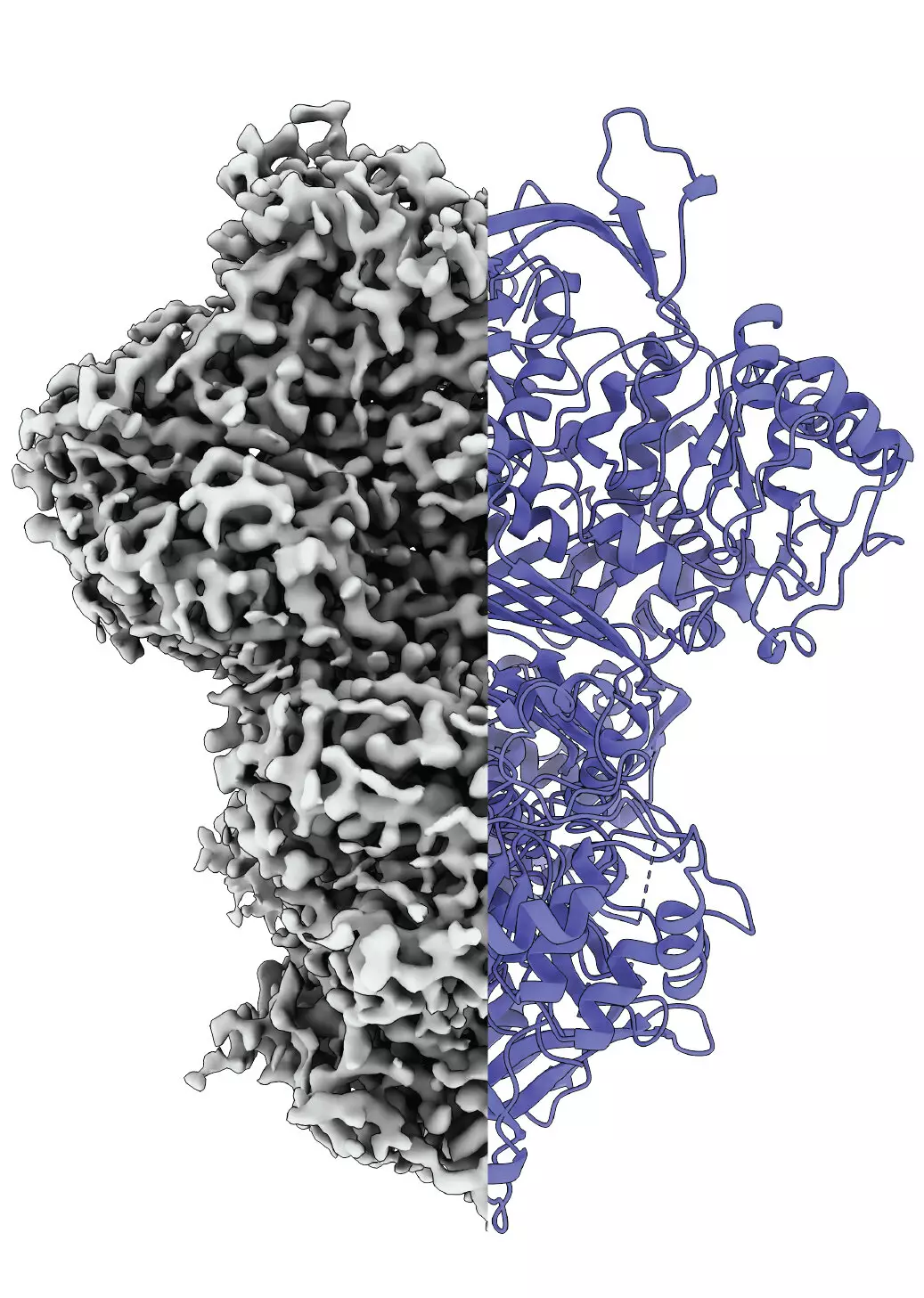
Proteins serve as the fundamental building blocks of life, orchestrating an array of biological processes crucial to the functioning of organisms. At the heart of protein functionality lies a significant principle: the structure of a protein intrinsically dictates its role within metabolic pathways. Misalignments or alterations in this structure can spell disaster for cellular activities, often leading to dire health consequences. One such protein, myo-inositol-1-phosphate synthase (MIPS), has recently taken center stage in a study that sheds light on protein dynamics, particularly how such proteins can exhibit multiple structural states, adapting to their immediate environment.
A pioneering research collaboration between scientists at Martin Luther University Halle-Wittenberg (MLU) and the National Hellenic Research Center in Greece has unveiled unprecedented insights into the MIPS protein. It was established that, unlike many proteins that harbor fixed structures, MIPS possesses a dynamic nature, transitioning between disordered and ordered states as it becomes active. Such fluctuations were observed using sophisticated cryo-electron microscopy techniques, which demonstrated that MIPS operates through at least three distinct states: a loosely organized disordered state, a structured ordered state, and a lesser-known intermediate state whose precise role remains to be deciphered.
This exploration into MIPS exemplifies the growing interest in understanding how proteins function in conditions that closely mimic their natural environments. Traditional methods often involve isolating proteins from their biological contexts, leading to a misrepresentation of their behavior. As Professor Panagiotis Kastritis aptly noted, the new methodology allows for the observation of proteins “under almost native conditions,” presenting a more accurate depiction of their functionality.
MIPS holds a vital role in synthesizing inositol, a compound often referred to as vitamin B8, which, while essential for cellular processes, is not classified as a true vitamin since the human body is capable of its production. This places MIPS within a critical metabolic pathway that not only influences inositol levels but also potentially affects numerous biochemical interactions and functions in human health.
The research conducted by Kastritis and his team has critical implications, illustrating how MIPS’s functionality can vary drastically depending on its structural state. Toni Träger, a member of the research group, emphasizes the connection between MIPS and the broader metabolic pathways, highlighting the potential impacts this research could have on therapeutic developments. By delineating the intricate workings of proteins like MIPS, scientists stand to better understand conditions arising from metabolic disorders and pave the way for innovative treatment approaches.
The exploration of MIPS was not a solitary endeavor; it sparked inquiry into a broader class of related proteins known as isomerases. An analysis of over 340 isomerases indicated that the intriguing behavior of MIPS may be a common characteristic among its relatives. Such findings not only enrich our understanding of protein biology but the patterns discovered could lead to significant advancements within the realms of biochemistry and pharmacology.
From a foundational perspective, these insights into MIPS represent a crucial stepping stone in the ongoing journey to comprehend protein functionalities. Addressing intricate questions surrounding the need for the mysterious intermediate state within the MIPS structure may lead to novel hypotheses regarding protein behavior under physiological conditions—questions that are ripe for future exploration.
The revelations regarding MIPS beckon the scientific community to reassess traditional methodologies and consider the benefits of embracing innovative, context-based approaches to protein analysis. As we deepen our understanding of proteins as dynamic entities—capable of structural adaptations that influence their functional capabilities—the potential for groundbreaking applications in biomedical fields becomes increasingly tangible. In closing, Kastritis’ research not only contributes valuable data to the world of protein science but also serves as a catalyst for further inquiry into the multifaceted realm of protein behavior. The journey to fully understanding MIPS and its relatives is only just beginning, raising an enticing prospect for scientists worldwide to contribute to this pivotal area of research.